ADVATE is a medicine used to prevent and control bleeding in adults and children with hemophilia A. ADVATE is not used to treat von Willebrand disease.
What is ADVATE?
ADVATE is a medicine used to replace clotting factor (factor VIII or antihemophilic factor) that is missing in people with hemophilia A (also called “classic” hemophilia). ADVATE is used to prevent and control bleeding in adults and children (0-16 years) with hemophilia A. Your healthcare provider (HCP) may give you ADVATE when you have surgery. ADVATE can reduce the number of bleeding episodes in adults and children (0-16 years) when used regularly (prophylaxis). ADVATE is not used to treat von Willebrand disease.1
In the hemophilia A community, no two patients are the same
When finding a factor VIII deficiency bleeding disorder treatment for hemophilia A, you and your healthcare provider should consider your individual needs.1 There are many characteristics that make you unique.1,2
![Each patient with hemophilia A is unique and has different needs, lifestyle, bleed frequency, personal goals—ADVATE [Antihemophilic Factor (Recombinant)] is a treatment option for hemophilia A.](/dist/images/style-guide/block-image.png)
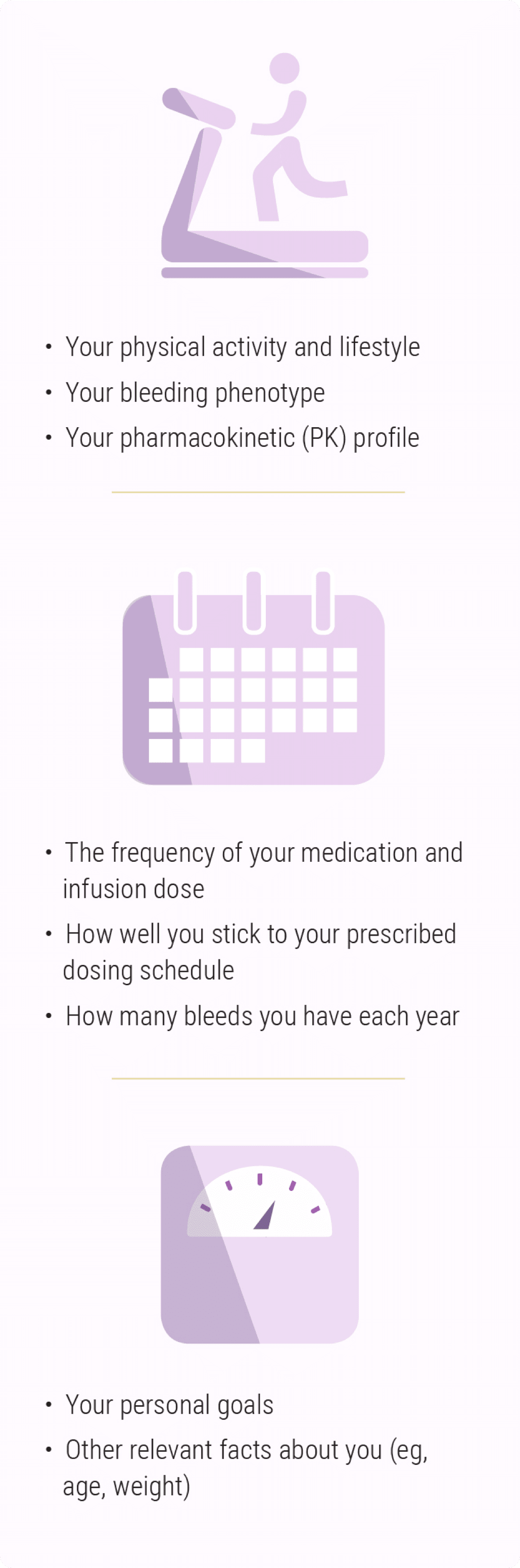
While living with hemophilia A will always be a part of your reality—both the welcome and unwelcome moments—you need treatment options that can help navigate the complexities of real life with hemophilia A.
What is a bleeding phenotype?
A patient’s bleeding phenotype is a collection of his or her bleed history, including bleeding disorder type and severity, annual bleed frequency, and overall joint health.3
What is PK?
Pharmacokinetics (or “PK”) is the science of how a drug is used up in the body.4 A person's PK profile helps determine how quickly or slowly his or her body breaks down treatment. Each person’s PK profile is different, and treatment can be personalized to your profile.5
About ADVATE
For adults and children with hemophilia A, ADVATE temporarily replaces the clotting factor VIII that's missing or low in the blood.6,7 In 2003, ADVATE became the first recombinant factor VIII treatment free of blood-based additives.7,8
What does recombinant factor VIII mean?
Today, clotting factor VIII concentrates can be made without human plasma. These types of infusions are called recombinant clotting factors. Recombinant factor VIII was one of the first treatments approved for hemophilia that didn't pose the threat of transmitting a blood-borne virus.6
A legacy of real patient experience
over
20
years
Over 20 years of treatment experience in the real world.7
over
43
billion
ADVATE is a global leader in factor VIII treatment, with over 43 billion International Units distributed globally.*8
*As of July 2022.
3
indications
ADVATE is indicated for prophylaxis, on-demand, and surgical use to help prevent bleeding episodes in children and adults.7
Interested in ADVATE?
A doctor discussion guide can help you have a productive conversation with your healthcare provider about your treatment options.
Committed to advancing hemophilia A care
Since introducing the first recombinant factor VIII treatment, Takeda has made advancements in dosing options, reconstitution with BAXJECT III®, and the introduction of myPKFiT® for personalized treatment.7,8

Explore ADVATE
safety information

Learn about health-related
quality-of-life data
References

- Valentino LA. Considerations in individualizing prophylaxis in patients with haemophilia A. Haemophilia. 2014;20(5):607-615.
- Petrini P, Valentino LA, Gringeri A, Re WM, Ewenstein B. Individualizing prophylaxis in hemophilia: a review. Expert Rev Hematol. 2015;8(2):1-10.
- Carcao M, Marijke van den Berg H, Ljung R. Correlation between phenotype and genotype in a large unselected cohort of children with severe hemophilia A. Blood. 2013;121(19):3946–3952.
- DiPiro J, Spruill W, Wade W, Blouin R, Pruemer J. Concepts In Clinical Pharmacokinetics. 5th ed. Bethesda, MD: American Society of Health-System Pharmacists; 2010.
- myPKFiT for Healthcare Professionals v3.3 User Manual. 2019.
- Treatment of Hemophilia. Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/hemophilia/treatment.html. Published 2020. Accessed May 18, 2020.
- ADVATE Prescribing Information.
- Takeda data on file.
